DAY 5 READING MEDICAL JOURNAL
Vasoplegia is the syndrome of pathological low systemic vascular resistance, the dominant clinical feature of which is reduced blood pressure in the presence of a normal or raised cardiac output. The vasoplegic syndrome is encountered in many clinical scenarios, including septic shock, post-cardiac bypass and after surgery, burns and trauma, but despite this, uniform clinical definitions are lacking, which renders translational research in this area challenging. We discuss the role of vasoplegia in these contexts and the criteria that are used to describe it are discussed. Intrinsic processes which may drive vasoplegia, such as nitric oxide, prostanoids, endothelin-1, hydrogen sulphide and reactive oxygen species production, are reviewed and potential for therapeutic intervention explored. Extrinsic drivers, including those mediated by glucocorticoid, catecholamine and vasopressin responsiveness of the blood vessels, are also discussed. The optimum balance between maintaining adequate systemic vascular resistance against the potentially deleterious effects of treatment with catecholamines is as yet unclear, but development of novel vasoactive agents may facilitate greater understanding of the role of the differing pathways in the development of vasoplegia. In turn, this may provide insights into the best way to care for patients with this common, multifactorial condition.
Background
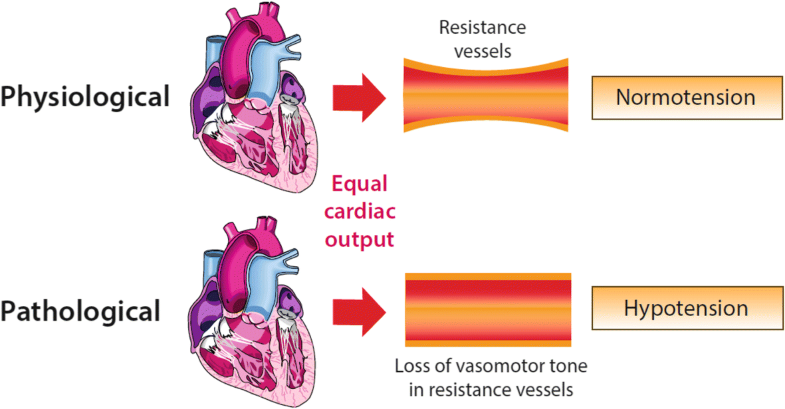
The relationship between tone in resistance vessels, under conditions of equal cardiac output, and the systemic blood pressure—preserved vasomotor tone leading to normotension and loss of vasomotor tone leading to hypotension
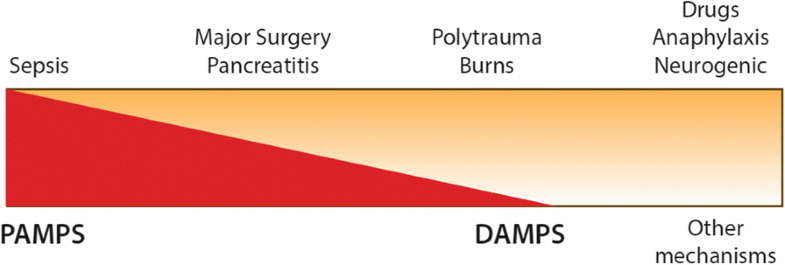
The main clinical causes of vasoplegia (top) and how they are perceived to relate to underlying aetiologies (bottom)—i.e. sepsis is predominantly a response to PAMPS (pathogen-associated molecular patterns) compared to burns or polytrauma where DAMPS (damage-associated molecular patterns) are the major cause
It is uncertain if it is justifiable to consider vasoplegia to be a pathophysiologically distinct entity representing uncontrolled failure of vascular homeostasis or to represent the end of a spectrum of vasodilatation.
Vasoplegic shock (VS), synonymous with distributive shock, is a more significant circulatory perturbation that is best described as vasoplegia with evidence of tissue hypoperfusion which may be accompanied with hyperlactataemia [5]. The presence of a raised lactate portends a particularly grave prognosis in the presence of shock or indeed septic shock [6, 7]. This review describes the key mechanisms involved in the development of VS, a process that is mediated by a diverse set of pathways which combine and contribute to the evolution of the shock state. Advancing our understanding of these pathways and their role in the transition from adaptive physiological to maladaptive pathological response may provide novel diagnostic tools, prognostic insights and therapeutic targets to guide the management of vasoplegia.
To date, our treatment options are limited and do not target some of the main pathophysiological pathways. First-line vasopressor therapy is typically with catecholamines and resistance is referred to as catecholamine-resistant hypotension (CRH). Although vasopressor infusion is required in order to maintain an adequate MAP, significant variation remains in clinical practice, particularly with regard to personalised targets depending on premorbid characteristics, and current research efforts are addressing this issue [8]. Moreover, it is well recognised that infused catecholamines are associated with a range of adverse effects on the metabolic, immune and coagulation systems [9, 10].
The tools available to clinicians to monitor the severity and impact of vasoplegia are limited [11, 12] and existing treatment goals may not result in the desired tissue level effects on microvascular flow [13]. Improved understanding of the pathophysiology of vasoplegia combined with new tools to monitor the impact of interventions on vessel function may lead to the development of the next generation of vasoactive therapies. The measurement of cardiac output, systemic blood pressure and central venous pressure allow derivation of the SVR, although targeting ‘normal’ values with insufficient consideration of their components may be hazardous.
CONCLUSIONS
Although vasoplegia is a well-recognised phenomenon, it still suffers from the lack of a unifying clinical definition. This prevents clinical trialists and translational scientists from sharing the common language necessary to facilitate research and increase understanding of this phenomenon. Certainly, we believe that a uniform approach to describing vasoplegia would reap benefits and stimulate further investigation of the underlying pathophysiological mechanisms. Vasoplegia is a complex phenomenon centred around vascular reactivity with multiple contributory potential mechanisms (outlined in Fig. 3). The advent of further alternatives to catecholamines, such as angiotensin II [97], may herald a new approach to treatment and the potential for alternative approaches—for further details, the reader is invited to consult the treatment article published in the same series. Optimum targets for systemic blood pressure remain contentious, and increasingly and appropriately, the pharmacological agents used to achieve these goals will be more closely scrutinised.
Comments
Post a Comment